Claim: Online article makes 3 claims about the management of COVID-19 and the deployment of vaccines in Australia
Source: Online article
Verdict: 1 FALSE, 1 MISLEADING, 1 TRUE
Researched by: Gifty Tracy Aminu
A news article being widely circulated on WhatsApp in Ghana claims the Australian government has admitted that COVID-19 vaccines are harming people and is offering up to $600,000 in compensation to seriously injured recipients of the vaccine.
The article published by an online platform, gqwaves.com on January 8, 2022, further claimed at least 79,000 Australians have reported severe side effects from COVID-19 vaccines.
“79,000 People! The government now ADMITS to severe vaccine side effects offering some victims over $600,000 in cash and compensation,” the article partly said.
This fact-check report would seek to verify;
- Whether the Australian government has said COVID-19 vaccines are harming people.
- Whether the Australian government is offering compensation to people with serious illnesses after taking the COVID-19 vaccines.
- Whether 79,000 people have reported severe side effects from taking the COVID-19 vaccines.
Claim 1
Australian government admits COVID-19 vaccines are harming people
Fact-check
“vaccination against COVID-19 is one of the most effective ways to reduce severe illness and death from infection,” the Australian Government Department of Health has said.
According to the health agency, vaccination against COVID-19 continues to play an important ongoing role in Australia.
“It is important to note that the overall benefits of a Therapeutic Goods Administration (TGA) approved COVID-19 vaccine outweigh the potential risks,” a spokesperson for the department of health said in an email when contacted by GhanaFact.
According to the government, 52,539,364 doses of COVID-19 vaccines have been administered throughout Australia as at February 14 ( more than 95% of people over the age of 16 have had at least one dose).
Verdict
This claim is rated FALSE.
Claim 2
Australian government is offering compensation to people with serious illnesses after taking the COVID-19 vaccines
Fact-check
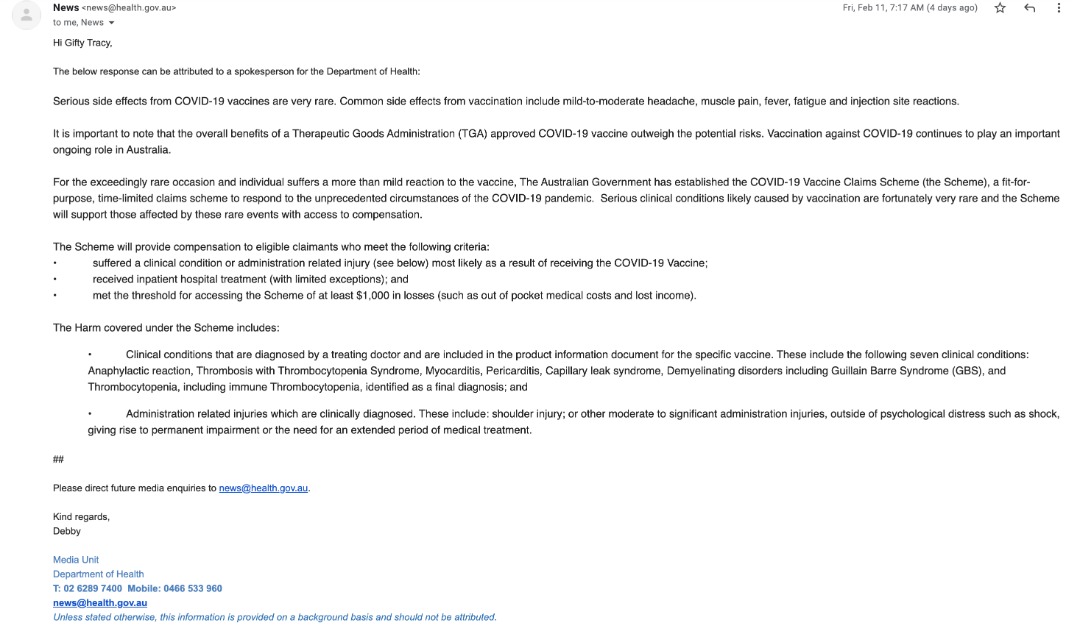
“Serious side effects from COVID-19 vaccines are very rare,” a spokesperson for the Australian Government Department of Health said in an email.
However, common side effects from vaccination include mild-to-moderate headache, muscle pain, fever, fatigue and injection site reactions.
For the exceedingly rare occasion when an individual suffers a more than mild reaction to the vaccine, the Australian Government said it had established the COVID-19 Vaccine Claims Scheme (the Scheme), a fit-for-purpose, time-limited claims scheme to respond to the unprecedented circumstances of the COVID-19 pandemic.
According to the government, the scheme will provide a simple, streamlined process to reimburse/compensate eligible people for their injuries without the need for complex legal proceedings.
The scheme will cover the costs of injuries $1,000 and above due to the administration of a Therapeutic Goods Administration (TGA) approved COVID-19 vaccine or due to an adverse event that is considered to be caused by a COVID-19 vaccination.
The list of adverse effects for claims purposes under the scheme includes the following clinical conditions that are diagnosed by a treating doctor and are included in the approved product information for the specific vaccine:
- anaphylactic reaction
- thrombosis with Thrombocytopenia Syndrome
- myocarditis
- pericarditis
- capillary leak syndrome
- demyelinating disorders including Guillain Barre Syndrome (GBS)
- Thrombocytopenia, including immune Thrombocytopenia, identified as a final diagnosis.
Harm not covered by the scheme includes:
- COVID-19
- psychological and psychiatric conditions (e.g. shock)
- secondary injuries (e.g. injury suffered when fainting, or a haematoma at the injection site that becomes infected)
- the following other injuries unless they form part of the symptom complex of a clinical condition listed above: headache; fatigue; injection site reaction; muscle or joint pain; dizziness; diarrhoea; pain in extremity; fever; insomnia; nausea; vomiting; lethargy; hyperhidrosis; chills; decreased appetite; malaise; lymphadenopathy; somnolence; abdominal pain; puritus; urticaria or rash; influenza-like illness; angioedema; anxiety-related reactions such as hyperventilation and fainting.
Verdict
The claim is rated TRUE.
Claim 3
79,000 people have reported severe side effects from taking the COVID-19 vaccines.
Fact-check
Australia has approved three COVID-19 vaccines to be administered in the country after they met the regulatory agency, Therapeutic Goods Administration(TGA)’s high standard of quality, safety and effectiveness.
These vaccines include Comirnaty (Pfizer), Spikevax (Moderna) and Vaxzevria (AstraZeneca).
Like any vaccine, COVID-19 vaccines can cause side effects, most of which are mild or moderate and go away within a few days on their own.
As shown in the results of clinical trials, more serious or long-lasting side effects are possible, the World Health Organization has said.
The medicine and therapeutic regulatory agency of the Australian Government, TGA closely monitors reports of suspected side effects (also known as adverse events) to the COVID-19 vaccines.
“We encourage people to report suspected side effects, even if there’s only a very small chance a vaccine was the cause. This provides valuable data that helps us identify trends or spikes that might reveal potential safety issues. Often, however, these events are not caused by the vaccines,” the Therapeutic Goods Administration(TGA) said.
According to TGA’s adverse report, as of November 7, 2021, a total of 78,880 adverse events following immunization have been recorded out of a total 36,773,837 doses of vaccines administered across the country at the time.
The breakdown of adverse effects according to the approved COVID-19 vaccines include;
- Vaxzevria (AstraZeneca) had 40,407 reports.
- Comirnaty (Pfizer) had 36,974 reports.
- Spikevax (Moderna) received 1,151 reports.
The most commonly reported reactions are:
- chest pain
- dizziness
- nausea
- headache
- fainting (syncope).
Verdict
This claim is rated MISLEADING because even though there have been reported cases of adverse events, the most commonly reported cases were mild and not severe as is being portrayed.



